Welcome to Ken-Hub!
An all-encompassing resource for contemporary technical advancements and trending subjects.


Science

Technology

Engineering

Management
Phases of Titanium
Titanium, a versatile and highly sought-after metal, has fascinated scientists, engineers, and enthusiasts alike with its remarkable properties and applications. Many might not realize that titanium undergoes various phases, each with unique characteristics and structures. In this article, we will delve into the fascinating journey of titanium through its different phases and explore the significance of these transitions in unlocking its extraordinary potential.
1. Alpha (α) phase of Titanium
The first phase in the life of titanium is the alpha phase, also known as α-Ti. At room temperature, titanium is stable in its α phase, which possesses a hexagonal close-packed (HCP) crystal structure. α-Ti exhibits excellent corrosion resistance, high strength-to-weight ratio, and biocompatibility, making it a popular choice in industries such as aerospace, medical implants, and marine applications. At elevated temperatures, the α phase of titanium, which possesses a hexagonal close-packed (HCP) crystal structure, becomes unstable. As a result, it undergoes a phase transformation into the β-phase, characterized by a body-centered cubic (BCC) crystal structure. This phase transition causes a significant reduction in the modulus of elasticity and stiffness of the titanium. The β phase of titanium, with its BCC structure, exhibits lower modulus of elasticity and stiffness compared to the α phase.
This change in mechanical properties at high temperatures is not desirable in certain applications, especially in the aerospace or thermal sectors. These industries often require materials that can maintain their structural integrity and mechanical performance under extreme conditions, including high temperatures. The decrease in modulus of elasticity and stiffness in the β phase can affect the load-bearing capacity and structural stability of titanium components in high-temperature environments. It can lead to increased deformation, reduced strength, and compromised performance under mechanical stress or thermal cycling. In aerospace applications, for example, where materials are exposed to elevated temperatures due to frictional heating or high-temperature operating conditions, maintaining structural integrity and dimensional stability is crucial. The α phase of titanium, with its higher modulus of elasticity and stiffness, provides superior mechanical properties that can withstand such demanding environments.
To address this issue, engineers and materials scientists explore various strategies to stabilize the α-phase at high temperatures. Alloying titanium with specific elements, such as aluminum or vanadium, can help maintain the α phase stability and prevent its transformation into the β phase. The binary phase diagram, illustrated in Figure 1, represents the relationship between the addition of an α stabilizer, such as aluminium, oxygen, nitrogen, or carbon, to titanium. In the diagram, the addition of oxygen to pure titanium results in a range of grades with increasing strength as the oxygen content increases. Aluminum, on the other hand, is the primary α stabilizer used commercially and is a significant component in most commercial titanium alloys. Figure 2 shows a schematic microstructure of typical α-Ti alloy, in which the white portion is α-phase and the black portion are the grain boundary β phase.


Figure 1. Effect of α stabilisers on Ti.
Figure 2. Schematic microstructure of α-Ti, with β-grain boundary
Aluminum is highly effective in strengthening the α phase of titanium, both at room temperature and elevated temperatures up to approximately 550°C. In addition to its strengthening properties, aluminum offers the advantage of low density, contributing to the overall lightweight nature of titanium alloys. However, there is a limitation to the amount of aluminum that can be added. This limitation arises due to the formation of a brittle titanium-aluminum compound when the aluminum content exceeds approximately 8% by weight.
By carefully controlling the composition of titanium alloys and considering the appropriate α stabilizers, engineers can tailor the strength and other mechanical properties of the material for specific applications. The binary phase diagram provides valuable insights into the effects of α stabilizers, such as aluminum and oxygen, on the microstructure and performance of titanium alloys. It is worth noting that other α stabilizers, such as nitrogen and carbon, also play a role in modifying the properties of titanium alloys. These elements can be utilized to enhance specific characteristics, such as hardness or corrosion resistance, depending on the desired application requirements.
Following are the mechanical properties of α-Ti.
-
Strength: The α phase of titanium exhibits high strength, making it suitable for applications that require excellent load-bearing capacity. It has a relatively high yield strength and tensile strength, contributing to its ability to withstand mechanical stresses.
-
Stiffness: Alpha titanium is known for its relatively high modulus of elasticity, which represents its stiffness. This property ensures that the material maintains its shape and resists deformation under external forces.
-
Ductility: The alpha phase of titanium has limited ductility, meaning it is less capable of undergoing large plastic deformation without fracturing. This characteristic can restrict its formability and may require additional processing techniques to shape the material.
-
Hardness: Alpha titanium possesses a moderate hardness, which provides it with resistance against wear and abrasion. However, it is not as hard as some other metals and may require surface treatments or coatings for specific applications.
2. Beta (β) phase of Titanium
When subjected to elevated temperatures, titanium undergoes a phase transition to the beta phase, or β-Ti. Beta titanium possesses a body-centered cubic (BCC) crystal structure, which is characterized by a more open lattice arrangement. This phase change results in increased ductility and improved formability, allowing for easier shaping and fabrication of titanium alloys. β-Ti alloys find applications in industries that require high-temperature performance, such as the automotive and chemical sectors. β-Ti, although stable at elevated temperatures, is metastable at room temperature. This means that over time, it tends to transform into the more thermodynamically stable alpha phase of titanium. This phase transformation from β to α titanium leads to a significant change in the material's mechanical properties. One of the notable effects of this phase transformation is the loss of ductility and formability in β-Ti at room temperature. Ductility refers to the ability of a material to undergo plastic deformation without fracturing, while formability relates to the ease with which a material can be shaped or formed into desired shapes and structures.
This loss of ductility and formability in beta titanium at room temperature can limit its applications, especially in scenarios where high formability or extensive shaping is required. To overcome this limitation, certain techniques can be employed to stabilize the β phase of titanium at room temperature. Several alloying elements can serve as β-stabilizers to maintain the stability of the beta phase in titanium alloys. Only a select few elements, including vanadium (V), molybdenum (Mo), niobium (Nb), iron (Fe), and chromium (Cr), are commonly utilized in significant quantities as β-stabilizers in titanium alloys. These elements can be divided into two categories: β-isomorphous elements and β-eutectoid elements.
β-isomorphous elements exhibit complete solubility with the β phase of titanium, meaning they can mix homogeneously with the β phase. As the concentration of these solute elements increases, the transformation temperature of the beta phase progressively decreases. Figure 3 illustrates a typical phase diagram depicting this behavior. The most important beta-isomorphous elements are Mo and V, although Nb and Ta have also found applications in specific alloy formulations. Figure 4 shows a schematic microstructure of typical β-Ti alloy, in which the white portion is grain boundary α-phase and the black portion are the β phase.
On the other hand, β-eutectoid elements have limited solubility in the β phase and form intermetallic compounds through eutectoid decomposition of the beta phase. Figure 5 represents a representative phase diagram for this type of system. β-eutectoid elements can be further categorized as sluggish or active. Fe, Cr, Mn, are commercially important sluggish metals. In the Ti-Fe, Ti-Cr, and Ti-Mn systems, the eutectoid decomposition of the β phase occurs at a slow rate, which means that intermetallic compound formation does not typically happen during standard fabrication, heat treatment, or service conditions. Therefore, for practical purposes, the behavior of Fe, Cr, Mn can be considered similar to that of beta-isomorphous elements. By carefully controlling the composition of the alloy and incorporating these β-stabilizers, engineers can tailor the mechanical properties and performance of the material to meet specific application requirements. It is important to note that the choice of β-stabilizers depends on several factors, including the desired alloy properties, processing conditions, and cost considerations. The aforementioned elements have proven to be effective in stabilizing the beta phase while imparting desirable characteristics such as improved strength, enhanced corrosion resistance, and elevated temperature stability.
Following are the mechanical properties of β-Ti.
-
Ductility and Formability: Beta titanium exhibits excellent ductility, making it highly formable and allowing for significant plastic deformation without fracture. This property enables the fabrication of intricate shapes and complex designs through various manufacturing processes such as forging, rolling, and extrusion.
-
Low Modulus of Elasticity: Beta titanium possesses a lower modulus of elasticity compared to alpha titanium. This characteristic imparts improved elasticity, making it useful in applications that require materials with high flexibility and resistance to deformation, such as springs and biomedical implants.
-
High Toughness: Beta titanium demonstrates exceptional toughness, allowing it to absorb a significant amount of energy before failure. This property makes it suitable for applications subjected to impact or cyclic loading, where resistance to crack propagation and improved durability are critical.
-
Corrosion Resistance: Beta titanium exhibits good corrosion resistance, especially in environments containing chloride ions. Although it may not possess the same level of corrosion resistance as alpha titanium, proper alloying and surface treatments can enhance its protective properties in corrosive environments.
3. α+β phase of Titanium
The α+β phase of titanium offers a dual-phase microstructure that combines the beneficial properties of both α and β phases. The resulting alloys exhibit a balance of strength, ductility, corrosion resistance, and thermal stability, making them valuable for a wide range of applications. From aerospace to biomedical and sports equipment, α+β titanium alloys continue to play a vital role in various industries where the unique combination of properties is essential for success. The α+β Ti includes α as well as β stabilizing agents to sustain the generally unstable α and β phases at high and low temperature respectively. Ti-6Al-4V, Ti-6Al-6V-2Sn, Ti-6Al-7Nb, and Ti62A, are some of the examples of α+β Ti alloy. The schematic microstructure shown in Figure 6 illustrates the typical arrangement of alpha (α) and beta (β) phases in a α+β titanium alloy for optical microscopy. It should be noted that in optical microscopy the α phase looks brighter and β phase are darker, while in electron microcopy the polarity reverses and α becomes darker and β becomes brigher. When examining the microstructure at high magnification (greater than 5000x), one can observe the presence of both α and beta phases in a sideplate or overlapping band format.
In some cases, due to a rapid cooling rate during processing, the β phase can undergo a transformation into a martensitic phase called alpha prime (α'). This transformed beta phase then precipitates on the α+β titanium matrix, creating distinct regions of α' within the alloy. Additionally, prior β phases, which may have transformed or remained in the β phase, can be observed within the α+β titanium matrix. These secondary phases are influenced by the thermal gradient experienced during cooling from the β-transus (882°C) temperature, which is the temperature at which the β phase transforms into the α phase.
The presence of these secondary phases, such as α' and retained β, can have a significant impact on the mechanical properties and performance of the α+β titanium alloy. The precise distribution and morphology of these phases within the microstructure influence factors such as strength, ductility, and fatigue resistance. Understanding and controlling the formation of these secondary phases is crucial for optimizing the microstructure and tailoring the alloy's properties for specific applications. Heat treatment processes, alloy composition adjustments, and cooling rates can be carefully manipulated to achieve the desired distribution and morphology of the α and β phases, as well as any secondary phases, in the α+β titanium alloy.
Following are the mechanical properties of α+β-Ti.
-
Strength: Alpha-beta titanium alloys demonstrate excellent strength, allowing them to withstand heavy loads and resist deformation. The presence of the beta phase contributes to the strength of the alloy, while the alpha phase provides additional reinforcement. The specific strength levels can vary depending on the alloy composition, heat treatment, and processing conditions.
-
Ductility: Alpha-beta titanium alloys exhibit good ductility, meaning they can undergo plastic deformation without fracturing. This property enables the alloys to be formed into complex shapes and undergo various fabrication processes, including forging, rolling, and extrusion. The ductility of these alloys is influenced by factors such as the proportion of alpha and beta phases and the presence of secondary phases.
-
Toughness: Alpha-beta titanium alloys possess high toughness, which refers to their ability to absorb energy and resist fracture under impact or high-stress conditions. This property is crucial for applications where the material needs to withstand sudden loads or impacts without failure. The combination of the alpha and beta phases, along with the presence of secondary phases, contributes to the toughness of the alloy.
-
Fatigue Resistance: Alpha-beta titanium alloys exhibit excellent fatigue resistance, enabling them to withstand cyclic loading without developing cracks or failure. This property is essential in applications where the material is subjected to repeated stress cycles, such as in aerospace components or structural elements. The microstructure, including the distribution of alpha and beta phases, plays a significant role in determining the fatigue resistance of the alloy.
-
Corrosion Resistance: Titanium alloys, including alpha-beta compositions, possess exceptional corrosion resistance. The oxide layer that forms naturally on the surface of titanium provides a protective barrier against corrosive environments, such as seawater or acidic solutions. This corrosion resistance is crucial for applications in marine, chemical, and biomedical industries where exposure to harsh environments is common.
-
Weldability: Alpha-beta titanium alloys exhibit good weldability, allowing them to be easily joined through various welding techniques. The presence of the beta phase enhances the weldability of these alloys compared to pure alpha titanium. Proper control of heat input and post-weld heat treatment can help preserve the mechanical properties of the welded joints and avoid the formation of detrimental phases.
Titanium, a metal that undergoes fascinating phase transitions, offers a unique combination of properties that make it an indispensable material in various industries. From the stable alpha phase to the more malleable beta phase and the versatile alpha-beta phase, titanium's phases provide a wide range of mechanical and chemical characteristics to meet diverse application requirements. Understanding and harnessing the potential of these phases allows engineers to unlock the extraordinary capabilities of titanium and pave the way for exciting advancements in the field of materials science
Bibliography and further readings:
-
Wang YZ, Ma N, Chen Q, Zhang F, Chen SL, Chang YA. Predicting phase equilibrium, phase transformation, and microstructure evolution in titanium alloys. Jom. 2005 Sep;57:32-9.
-
Tan X, Kok Y, Toh WQ, Tan YJ, Descoins M, Mangelinck D, Tor SB, Leong KF, Chua CK. Revealing martensitic transformation and α/β interface evolution in electron beam melting three-dimensional-printed Ti-6Al-4V. Scientific reports. 2016 May 17;6(1):26039.
-
Oh ST, Woo KD, Kim JH, Kwak SM. The effect of Al and V on microstructure and transformation of β phase during solution treatments of cast Ti-6Al-4V alloy. Korean Journal of Metals and Materials. 2017 Mar 3;55(3):150-5.
-
Fang ZZ, Paramore JD, Sun P, Chandran KR, Zhang Y, Xia Y, Cao F, Koopman M, Free M. Powder metallurgy of titanium–past, present, and future. International Materials Reviews. 2018 Oct 3;63(7):407-59.
-
Li X, Zhu Q, Liu S, Li F, Chen F, Wang H, Chang H. Phase transformation and microstructure evolution of Ti6Al4V-0.55 Fe alloy with different initial microstructure during continuous heating. Journal of Materials Research and Technology. 2022 May 1;18:1704-16.
-
Liu S, Shin YC. Additive manufacturing of Ti6Al4V alloy: A review. Materials & Design. 2019 Feb 15;164:107552.
-
Mishra A, Paul AR, Mukherjee M, Singh RK, Sharma AK. Evaluation of Cu-Ti dissimilar interface characteristics for wire arc additive manufacturing process. Rapid Prototyping Journal. 2023 Jan 27;29(2):366-77.
-
Mishra A, Paul AR, Mukherjee M, Singh RK. Bimetallic Structure of Ti6Al4V/IN718 with CuSi Interlayer for Wire-Arc Directed Energy Deposition Process. Metals and Materials International. 2023 Feb 18:1-4.
-
Mishra A, Paul AR, Sharma R, Mukherjee M, Singh RK. Interfacial characteristics of Ti6Al4V-IN718 dissimilar structure developed by wire-arc additive manufacturing using Monel-400 as an interlayer. Materials Today: Proceedings. 2023 Jan 1;80:241-7.
-
Li H, Zhao Z, Ning Y, Guo H, Yao Z. Characterization of microstructural evolution for a near-α titanium alloy with different initial lamellar microstructures. Metals. 2018 Dec 10;8(12):1045.

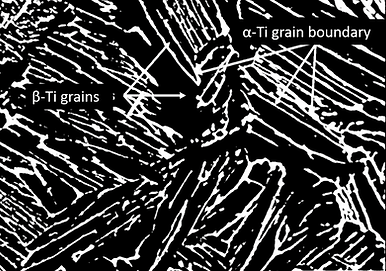

Figure 3. Effect of β-isomorphous stabilisers on Ti.
Figure 4. Schematic microstructure of β-Ti with grain boundary α.
Figure 5. Effect of β-eutectoid stabilisers on Ti.

Figure 6. Schematic microstructure of α+β titanium.